Nitrates, one of the most widespread water pollutants globally, pose a severe threat to both drinking water safety and human health. The electrochemical conversion of nitrates to ammonia not only reduces nitrate pollution but also generates valuable ammonia. This provides a sustainable pathway for restoring the global nitrogen cycle balance and offers technological support in terms of environmental and economic impacts for sustainable ammonia synthesis. However, the key challenge in this field of research lies in the development of electrode materials with low cost, high activity, and selectivity advantages. Li Miao's Research Team has addressed the bottleneck challenge in practical applications, caused by the weak adsorption energy between carbon active sites and reactant molecules, affecting reaction kinetics and thus removal efficiency and selectivity. They innovatively enhanced the microstructure control theory and methods at the heterojunction interface, focusing on the carbon activation sites and their weak affinity to reactant molecules. Through nitrogen doping, a catalyst with new active sites was constructed, activating adjacent carbon atoms and enhancing the electron transfer from metal to carbon. This resulted in significantly increased catalytic activity. The team developed a nitrogen-doped carbon-iron heterostructure (Fe@N10-C) electrocatalyst derived from a metal-organic framework (MOF) material. This innovative catalyst is applied in the electrochemical removal of nitrates and ammonia energy production. In the study, the efficiency of nitrate removal reached 125.8±0.5mgNgcat-1 h-1, and the ammonia selectivity was nearly 100% (99.7±0.1%), marking the highest values reported in existing research.
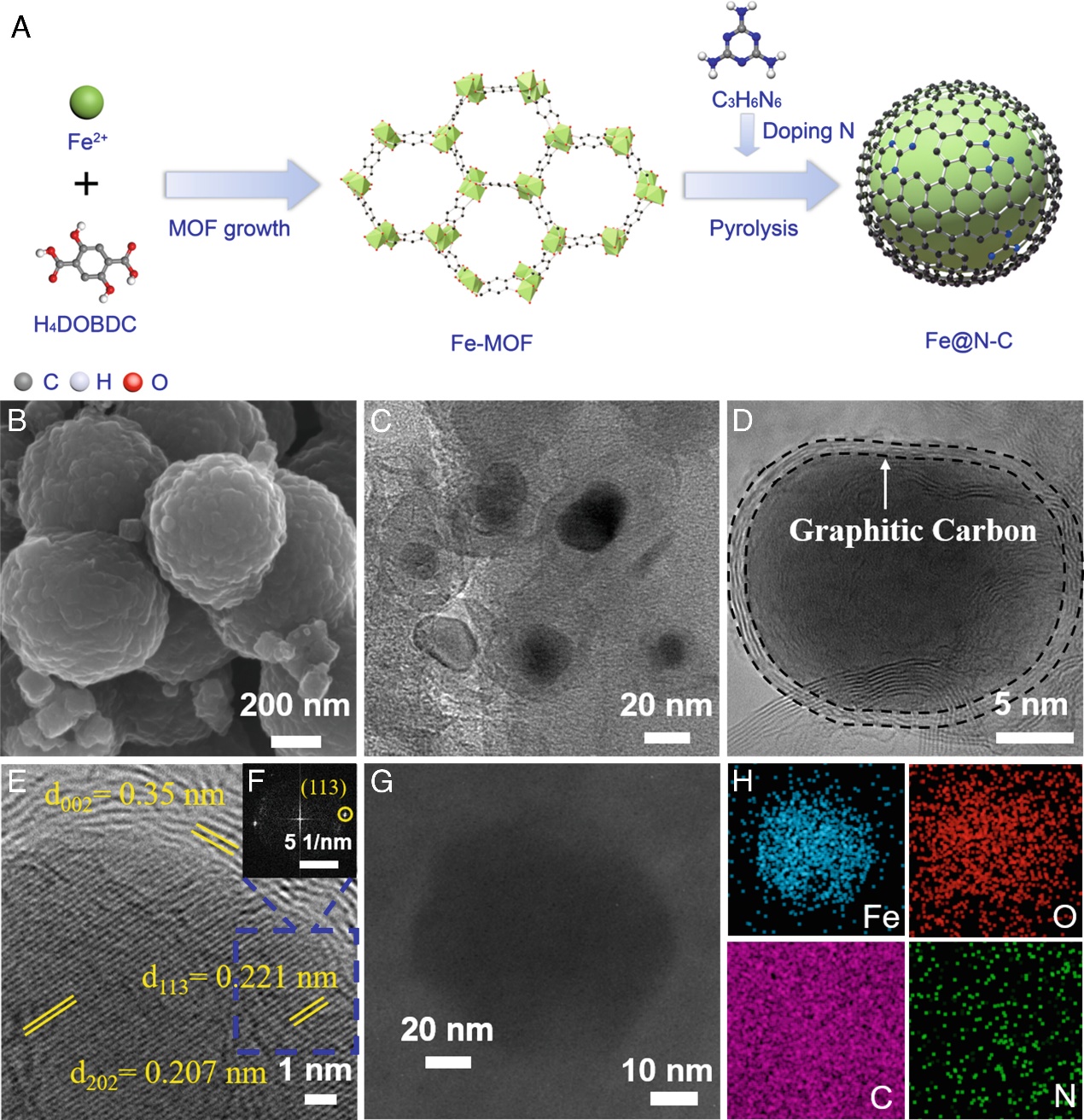
Figure 1: Schematic illustration, morphology, and elemental characterization of the Fe@N10-C catalyst."
The study utilized extended X-ray absorption fine structure spectroscopy to analyze the valence states and coordination environments of Fe, N, and C sites in the Fe@Nx-C catalyst, further confirming the formation of nitrogen-doped carbon structures during pyrolysis. Post-pyrolysis, the predominant nitrogen species in the Fe@Nx-C catalyst was identified as graphitic nitrogen. The primary active sites in Fe@Nx-C were carbon active sites adjacent to nitrogen sites (CN). The enhanced catalytic activity of Fe@N10-C was attributed to the appropriate nitrogen doping in the carbon layer surrounding the Fe nanoparticles.

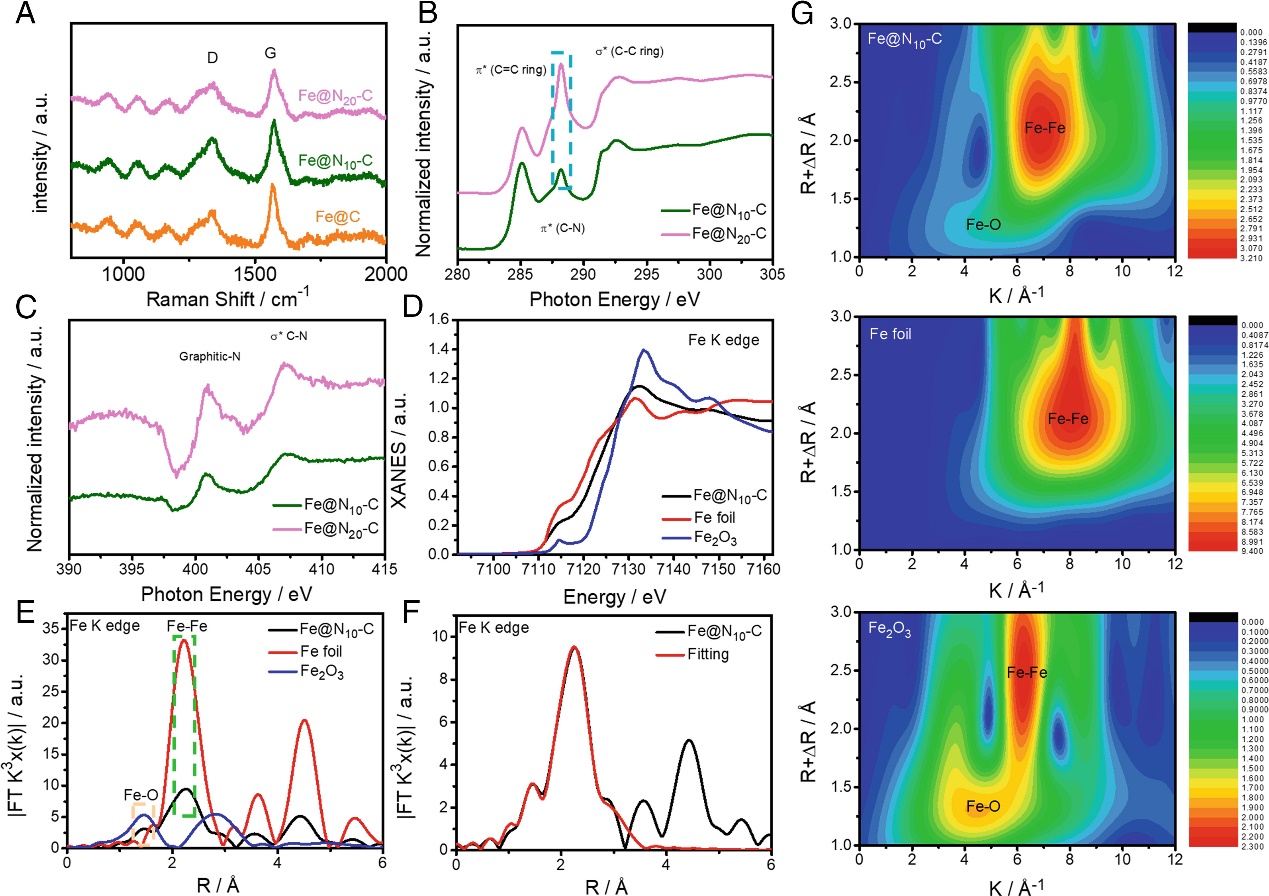
Figure 2: Structural Characterization of Fe@N10-C
To delve into the role of nitrogen in activating carbon atoms and enhancing the electrochemical nitrate reduction activity of Fe@N10-C, this study employed density functional theory calculations. Active sites of three representative models, Fe@N, Fe@N10-C, and Fe@N20-C, were revealed through difference charge plots, confirming charge transfer at the CN sites and distortion of neighboring carbon atoms upon N doping. Subsequently, the impact of N doping on the electronic structure was investigated, showing more pronounced charge accumulation at CN sites, indicating increased charge density facilitating NO3- adsorption. Bader charge analysis further affirmed greater charge transfer at CN sites, highlighting them as active sites. To better comprehend the activation of carbon atoms and the origin of Fe@N10-C catalytic activity, the interaction between Fe and N-doped carbon was explored. Difference charge plots demonstrated that N doping influenced the electron transfer from Fe NPs to N-doped carbon, activating C atoms, subsequently affecting reactant molecule adsorption and improving reaction kinetics.
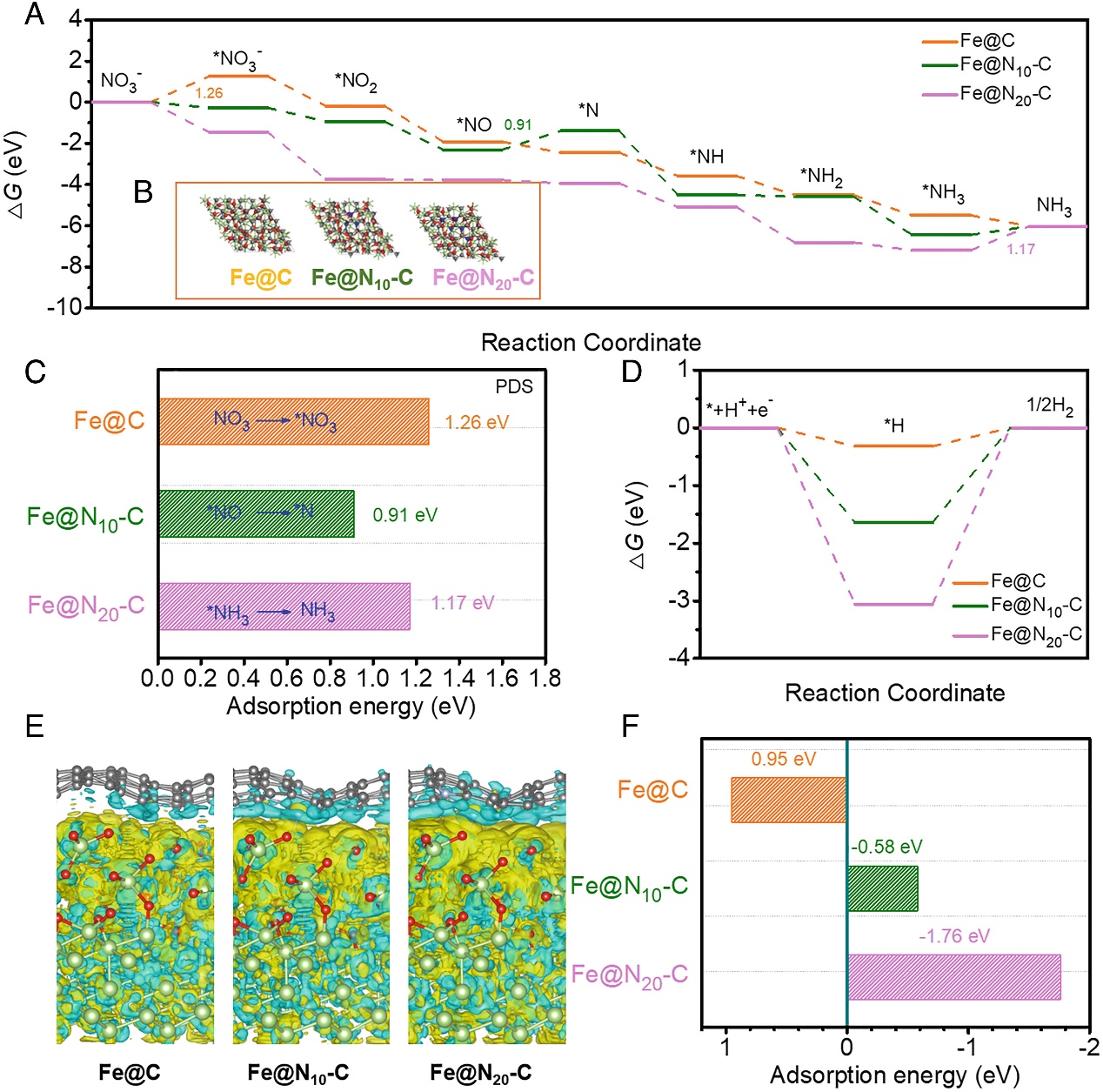
Figure 3: Schematic Diagram of the Reaction Mechanism
The research findings with the title N-doped carbon-iron heterointerfaces for boosted electrocatalytic active and selective ammonia production have been published online in the Proceedings of the National Academy of Sciences of the United States of America.
The first author of the paper is Zhang Shuo, a Ph.D. candidate from the School of Environment at Tsinghua University, Class of 2019. The corresponding author is Associate Professor Li Miao from the School of Environment at Tsinghua University, and Professor Liu Xiang from the same school provided crucial guidance and assistance in experimental research and analysis. The research project received support from the general project and the key research and development program of the National Natural Science Foundation of China (NSFC).
Link to the paper: https://doi.org/10.1073/pnas.2207080119
Contributed by: Division of Soil and Groundwater Environment





