Prof. Bailong Xiao’s and Xueming Li’s groups demystify a force-transduction protein machinery
January 22nd, 2018, the research groups led by Prof. Bailong Xiao (School of Pharmaceutical Sciences) and Prof. Xueming Li (School of Life Sciences) at Tsinghua University solved the structure of the biological force sensor -the mechanosensitive Piezo1 channel - and proposed a sophisticated lever-like mechanotransduction mechanism for explaining its extraordinary ability to convert mechanical force into cation conduction. The findings, described in a paper entitled “Structure and mechanogating mechanism of the Piezo1 channel”, were published online in the journal Nature.
As we touch our skin for shaking hands, hugging or kissing, the gentle force effectively excites our sensory neurons to elicit the pleasant touch sensation. Or as blood flows through our vessels, the cells that constitute these vessels responds to the shear stress of blood flow to ensure normal circulation. All these essential biological functions rely on the evolutionarily conserved Piezo family of proteins, including Piezo1 and Piezo2, which were originally identified by Dr. Ardem Patatpoutian’s lab at the Scripps Research Institute in 2010. In humans, mutations of Piezo1 or Piezo2 genes have also been linked to genetic diseases, stressing their functional importance.
Piezo proteins are large and complex transmembrane proteins that don’t possess notable sequence homology with any known class of ion channels. How do Piezo proteins exactly function at the molecular level to serve as effective force-transduction molecules?
Dr. Bailong Xiao began to address this question when he was still a postdoctoral fellow in Patapoutian lab. Together with his collaborators, they demonstrated that purified Piezo proteins are able to conduct cations by themselves, thus for the first time establishing them as the long-sought-after mammalian mechanosensitive cation channels. This groundbreaking work was published in Nature in 2012, and Dr. Bailong Xiao was co-first author. Notably, the Web of Science has listed this paper as a highly cited article.
But fundamental questions remained unanswered, among them: How do these proteins three-dimensionally organize into mechanosensitive channels? How do they conduct ions in response to force stimulation?
After setting up his own lab at Tsinghua University in 2013, Dr. Bailong Xiao continued his effort to tackle these questions, and made significant breakthroughs.
In a paper the group published in Nature in 2015, along with collaborators at the university, they reported resolving a medium-resolution cryo-electron microscopy (cryo-EM) structure of the full-length mouse Piezo1. Remarkably, they found that Piezo1 trimerizes to form a three-bladed, propeller-like structure with a central ion-conducting pore. However, the resolution of this structure is not high enough to allow them to assign specific amino-acid residues into the structure.
In a paper published in Neuron in 2016, his group functionally identified the bona-fide ion-conducting pore and key pore-property-determining residues. They proposed that Piezo1 might employ a separate pore module for ion conduction and mechanotransduction module for force sensing and transduction.
In a paper published in Nature Communications in 2017, his group identified Piezo1 interacting proteins - the Sarco/Endoplasmic Reticulum Ca2+ ATPase (SERCA). Interestingly, they found that SERCA binds right to a short linker that connects the mechanotransduction module and the pore module, which might prevent the functional coupling of the two modules and therefore inhibit the mechanical activation of Piezo1.
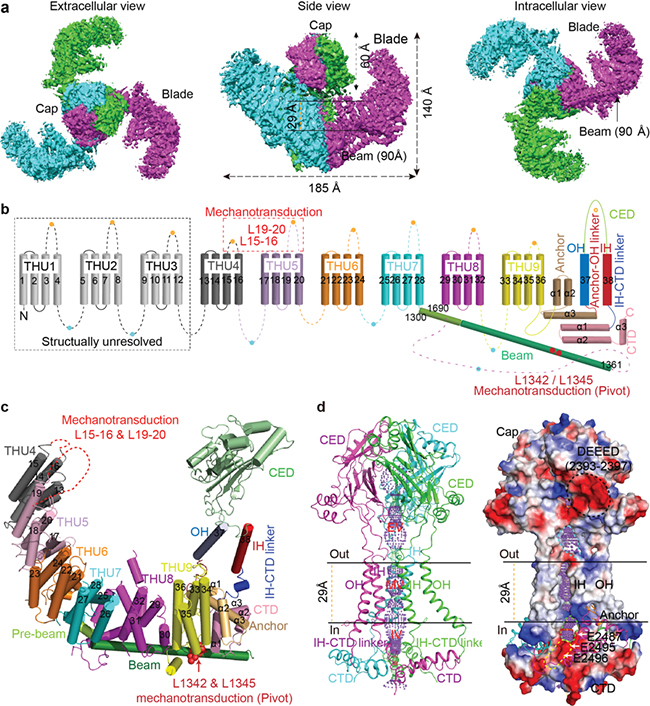
a, The three-bladed, propeller-like cryo-EM structure of the Piezo1 ion channel.
b, 9 repetitive THUs and the 38-TM topology model.
c. A cartoon model showing one subunit with featured structural domains labeled.
d. The ion-conducting pore module shown in a Ribbon diagram or with surface electrostatic potential.
The functionally identified regions and residues critical for mechanical activation of Piezo1 are shown in figs. b and c.
In this new study, they have made a major breakthrough in pushing the three-bladed, propeller-like Piezo1 structure into a much higher resolution (Fig. 1a), allowing them to see more structural features and assign amino-acids into key domains. Dr. Bailong Xiao believes that the new structure clearly helps to reveal some unique features that might be critical for Piezo1 to function as a sophisticated mechanotransduction channel.
Firstly, despite the lack of sequence repetition, they identified 9 repetitive units constituted of 4 TMs each (Figs. b, c), and named this structural unit as THU (Transmembrane Helical Unit). Together with the last two TMs, which are termed outer helix (OH) and inner helix (IH), Piezo1 possesses an unprecedented 38-TM topology with a total of 114 TMs in the trimeric channel complex.
Secondly, they found that the peripheral 9 THUs in each subunit are organized into the highly curved blade structure (Figs. a-c), which means that Piezo1 might be able to curve its residing membrane. Furthermore, they observed that an intracellular helical layer exists immediately underneath the membrane (Fig. c), which might help to stabilize the curved TM blade in the membrane.
Thirdly, three 90 ampere-long intracellular beam-like structures connect the three peripheral blades to the pore via the interfaces of the C-terminal domain, anchor-resembling domain and outer helix (Figs. a-c). They hypothesize that Piezo1 might employ the beam to form a lever-like apparatus for transducing force from the peripheral blade to the central ion-conducting pore.
They also observed unique features of the central ion-conducting pore, which is likely in a closed conformation (Fig. d). Three IH enclose a hydrophobic transmembrane pore, which is not completely sealed from the membrane. This feature raises an intriguing possibility that membrane lipids may affect the ion permeation and gating of Piezo1. Interestingly, both the extracellular vestibule (EV) and intracellular vestibule (IV) have large fenestration sites immediately above and below the membrane, respectively. Considering their previously identified key pore-property-determining residues, they propose that cations might enter through the extracellular fenestration sites and exit through the three intracellular fenestration sites and the connecting side portals.
To gain further understanding of how the central pore is effectively gated by the peripheral blade and beam structures, they first analyzed Piezo1 structures in distinct motion states, and revealed long-distance mechanical motions that are potentially associated with the mechanogating process. Notably, the beam displays uneven movement with large motion at the distal beam while subtle movement at the proximal end. As a whole, the motion feature of the peripheral blade and the beam is reminiscent of a lever apparatus.
Then they carried out extensive mutagenesis, biochemical and electrophysiological studies to identify key domains and residues critical for the mechanical activation of Piezo1. In line with their hypothesis, they found that deletions of extracellular loops in the distal THUs or mutations of two residues (L1342/L2345) at the proximal end of the beam severely affected the mechanical activation of Piezo1.
On the basis of these structural and functional characterizations, they proposed that Piezo1 might employ its characteristically curved blades and the long beams with the L1342/L2345 as a pivot to form a lever-like apparatus. Such a lever-like mechanotransduction mechanism might enable Piezo channels to effectively convert a large conformational change of the distal blades to a relatively slight opening of the central pore, allowing cation-selective permeation. Three sets of such lever-like apparatus are further assembled into a gigantic propeller-like machinery, which might confer a coordinated mechanosensitivity.
Dr. Bailong Xiao believes that this study provides a fundamental understanding of how Piezo proteins are elegantly designed to fulfill their designated function as specialized mechanotranduction channels for effectively transducing mechanical force into a biological signal, and also paves the way for targeting Piezo1-based human diseases, such as the dehydrated hereditary stomatocytosis and congenital lymphedema.
In this study, Drs. Bailong Xiao and Xueming Li are the co-corresponding authors; Qiancheng Zhao, Heng Zhou, Shaopeng Chi and Yanfeng Wang are co-first authors; Jianhua Wang, Jie Geng, Kun Wu, Wenhao Liu and Tingxin Zhang, Dr. Meng-Qiu Dong and Dr. Jiawei Wang are co-authors. This work was supported by grants from the National Natural Science Foundation of China and the National Key R&D Program of China.
Link:https://www.nature.com/articles/nature25743
Contributor: School of Pharmaceutical Sciences
Editor: Guo Lili

