Professor Yigong Shi’s Group Reported Three-dimensional Structure of Human g-secretase in Nature
Dr. Yigong Shi’s laboratory in School of Life Sciences, Tsinghua University, published an Article entitled “Three-dimensional structure of human g-secretase” in Nature on June 29, 2014. In this article, they reported the cryo-EM structure of human g -secretase at 4.5 Å resolution, which provides an important framework for understanding the function and disease mechanism of the g-secretase complex and Alzheimer’s Disease.
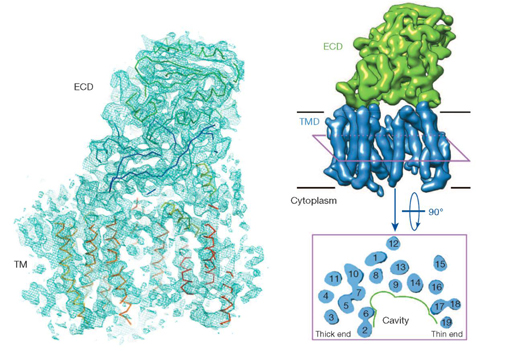
The g-secretase, comprising presenilin 1 (PS1), Pen-2, Aph-1 and Nicastrin, is a membrane-embedded protease complex that controls a number of important cellular functions through substrate cleavage. One of the best studied substrates of g-secretase is the amyloid precursor protein (APP). Aberrant cleavage of APP results in aggregation of amyloid-b peptide, accumulation of which in the brain is suggested to be the cause of Alzheimer’s Disease.
Structural characterization of g-secretase has been extremely slow due to the daunting technical challenges involving expression and purification of the intact g-secretase complex. During the past several years, Shi Lab has launched rigorous and systematic efforts to obtain homogeneous and active human g-secretase for structural investigations. They finally succeeded in transient co-expression of all four components of the human g-secretase complex in mammalian HEK293 cells.
In collaboration with Dr. Xiaochen Bai in Dr. Sjors Scheres’ laboratory at Molecular Research Council at Cambridge, UK, they employed cryo-electron microscopy (cryo-EM) single-particle reconstruction. By exploiting the technological advances in direct electron detection and statistical image processing, the three-dimensional structure of an intact human g-secretase complex was obtained at 4.5 Å resolution. The g-secretase complex comprises a horseshoe-shaped transmembrane domain, which contains 19 transmembrane segments (TMs), and a large extracellular domain (ECD) from Nicastrin, which sits immediately above the hollow space formed by the TM horseshoe. Intriguingly, nicastrin ECD is structurally similar to a large family of peptidases exemplified by the glutamate carboxypeptidase PSMA.
This study for the first time reveals the domain architecture, secondary structural elements, TM arrangement, and ECD fold of g-secretase, and marks an important step towards elucidating the molecular mechanisms of this key enzyme whose aberrant activity engenders the Alzheimer’s disease.
This work was supported by funds from National Natural Science Foundation of China projects 30888001, 31130002 and 31021002, the Ministry of Science and Technology of China project 2009CB918801, a European Union Marie Curie Fellowship, and the UK Medical Research Council MC_UP_A025_1013. Peilong Lu, Ph.D. student from Tsinghua University, Dr. Xiao-chen Bai, postdoc from MRC Laboratory of Molecular Biology and Dan Ma, Ph.D. student from Tsinghua University, are co-first authors.
The paper link: http://www.nature.com/nature/journal/vaop/ncurrent/full/nature13567.html

