Jijie Chai Research Group from Tsinghua Published Article in Science, Revealing Molecular Mechanisms of Plant Innate Immunity
The research group led by Prof. Jijie Chai from School of Life Sciences, Tsinghua University, published a paper in Science online on October 10, 2013. The paper entitled "Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex” reported the crystal structures of FLS2LRR-Flg22-BAK1LRR, revealing the molecular mechanisms of the complex activation.
Both animal and plant innate immune systems relies on detection of conserved signature components of pathogens, termed Pathogen-Associated Molecular Patterns (PAMPs), for stimulating anti-microbial responses. In plants, perception of PAMPs is mainly mediated by the membrane-localized pattern recognition receptors (PRRs). Upon recognition of PAMPs, PRRs initiate an array of shared immune responses, leading to PAMP-triggered immunity (PTI).
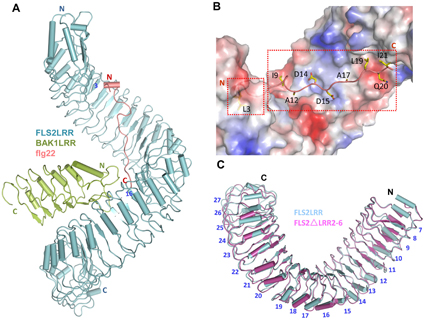
Fig. Ectodomains mediate the flg22-induced hetero-dimerization of FLS2 and BAK1.
(A) Overall structure of FLS2LRR-flg22-BAK1LRR. (B) Flg22 binds to a shallow groove at the inner surface of the FLS2LRR solenoid. (C) Structural comparison of the ligand-bound FLS2LRR with the free FLS2△LRR2-6.
Flagellin important for bacterial motility is a typical PMAP. Flagellin-sensitive 2 (FLS2) is the first PRR identified in Arabidopsis and has a critical role in plant immunity by recognizing flg22 (the epitope of bacterial flagellin). Within seconds of binding, flg22 triggers FLS2 heteromerization with BRI1-associated kinase 1 (BAK1) and their reciprocal activation followed by plant immunity. In addition to FLS2, BAK1 also forms ligand-dependent heterodimerization with several other RLKs such as EFR and PEPR1/2 for immune responses. The FLS2-mediated PTI signaling pathway acts as a paradigm for understanding the molecular mechanisms underlying plant innate immunity. Many studies have been performed to investigate the FLS2-flg22 interaction, but it remains undefined how the peptide is recognized by FLS2 and consequently activates FLS2.
Prof. Jijie Chai’s Research Group solved the crystal structure of FLS2 and BAK1 ectodomains complexed with flg22. The structure reveals that a conserved and a nonconserved site from the FLS2 solenoid recognize the C- and N-terminal segment of flg22, respectively, without oligomerization or conformational changes in the FLS2 ectodomain. Besides directly interacting with FLS2, BAK1 acts as a co-receptor by recognizing the C terminus of the FLS2-bound flg22. Through collaboration with researchers from Dr. Jianmin Zhou’s and Dr. Cyril Zipfel’s groups, the mechanisms of FLS2 activation by flg22 were further validated by in vitro and in vivo experiments. In addition to providing a model for understanding plant PTI, the data from these studies make it possible to engineer FLS2 to develop broad spectrum disease-resistant crop varieties.
Prof. Jijie Chai and Dr. Zhifu Han from School of Life Sciences, Tsinghua University, and Dr. Jianmin Zhou from Institute of Genetics and Developmental Biology are the corresponding authors of the paper; Yadong Sun (PhD student) from School of Life Sciences, Tsinghua University, and Lei Li (PhD student) from CAS Institute of Genetics and Developmental Biology are co-first authors; Prof. Cyril Zipfel and Dr. Alberto P. Macho from the Sainsbury Lab in Norwich Research Park also participated in part of the work. Shanghai Synchrotron Radiation (SSRF, Beam line BL17U1) provided support for diffraction data collection. This research in Tsinghua University was funded by the National Outstanding Young Scholar Science Foundation of China (grant no. 31025008) and Chinese Natural Science Foundation Grant (grant no. 31130063).
The paper links:http://www.sciencemag.org/content/early/2013/10/09/science.1243825

